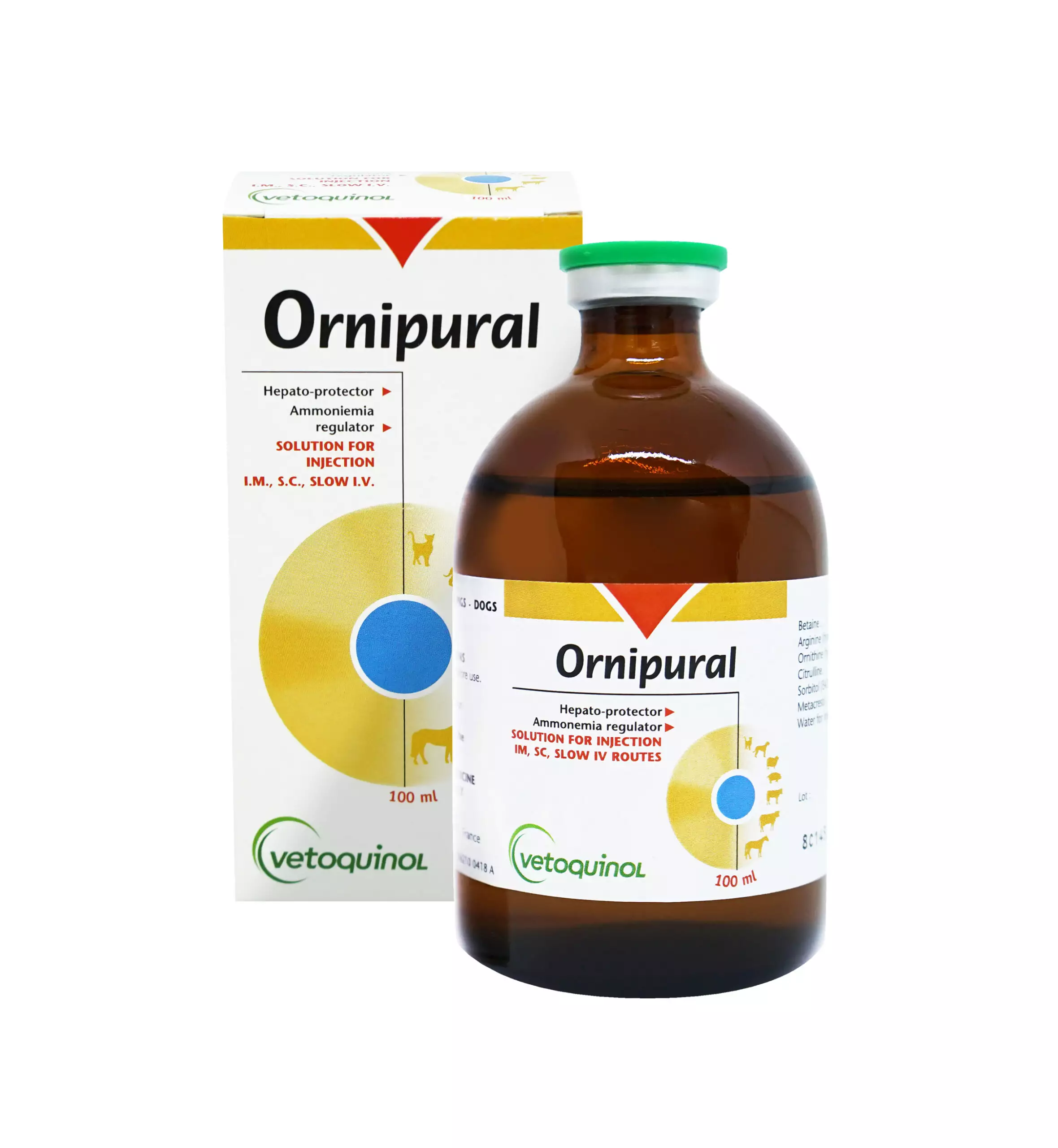
Buy Ornipural Online
$50.00
In the case of concomitant oral administration of cations (aluminium, calcium, iron, magnesium),
the bioavailability of Marbofloxacin can be reduced. It is recommended to lower the dose of
Theophylline in the case of concomitant administration.
ORNIPURAL
Buy Ornipural Solution
Stimulation of hepato-digestive activity in case of digestive disorders and hepatic impairment.complete injectable solution combining hepatoprotective, lipotropic and diuretic active ingredients (betaine, sorbitol, ornithine, citrulline and arginine).
Dosage and administration:
50 to 100 ml in adult cattle (I.M., S.C. or slow I.V. administration).
Withdrawal period:
meat: zero days
Milk: zero days
Ornipural Solution
Presentation:
100 ml vial
Description
PHARMACODYNAMIC PROPERTIES
Marbofloxacin is a bactericidal anti-infectious synthesis belonging to the fluoroquinolones group.
It acts by inhibition of DNA-gyrase. Its very broad spectrum is directed towards Gram positive
(especially Staphylococcus and Streptococcus), Gram negative bacteria (Escherichia Coli,
Salmonella Aertricke, Citrobacter freundii, Aerobacter cloacae, Serratia marcescens, Morganella
morganii, Proteus sp, Klebsiella sp, Shigella sp, Pasteurella sp, Haemophilus sp, Moraxella sp,
Pseudomonas sp, Brucella canis) and Mycoplasma.
PHARMACOKINETIC PARTICULARS
After oral use at the recommended dose of 2 mg/kg in the cat and dog, Marbofloxacin is rapidly
absorbed and reaches maximal plasmatic concentrations of 1.5 µg/ml in 2 hours approximately.
The bioavailability of Marbofloxacin is close to 100 %. It is slightly related to plasmatic proteins (
than 10 %) and is widely distributed in the entire organism. In the majority of tissues (liver,
kidney, skin, lungs, bladder and digestive tract), tissular concentrations are higher than those of
plasma. Marbofloxacin is slowly eliminated (half-life of elimination : 14 hours in the dog and 10
hours in the cat) and mainly in active form in the urines (2/3) and faeces (1/3).
Contra-indications
Marbofloxacin is well tolerated in growing medium sized-dogs until doses reaching 4 mg/kg/day
for 13 weeks. However, it is not recommended to use it in giant breeds dogs under 1 year.
Side effects
Minor side effects may occur during treatment, such as vomiting, softening of saddles, alteration
of the thirst, transitory hyperactivity. These signs disappear spontaneously and it is not
necessary to stop treatment.
Use during pregnancy and lactation
Studies in animals of laboratory (rat, rabbit) have not produced any evidence of a teratogenic,
foetotoxic or maternotoxic effect of Marbofloxacin at the dose used in therapeutics.
Harmlessness of the product in queens during pregnancy and suckling has not been shown. The
use of this product in pregnant or suckling females should be avoided or only accordingly to the
benefit/risk assessment by the responsible veterinarian.
Interaction
Reviews
There are no reviews yet.
Related products
Oral
Oral





Be the first to review “Buy Ornipural Online”